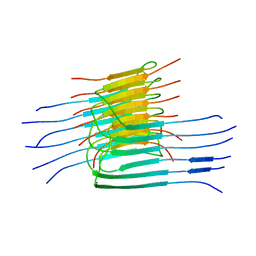
Properties
| Input source #1: NMR data (NEF) - Assigned chemical shifts, Distance restraints, Dihedral angle restraints, RDC restraints | combined_18127_2lmn.nef |
| Input source #2: Coordindates | 2lmn.cif |
| Diamagnetism of the molecular assembly | True (excluding Oxygen atoms) |
| Whether the assembly has a disulfide bond | None |
| Whether the assembly has a other bond | None |
| Whether the assembly contains a cyclic polymer | None |
| Overall data processing status | Warning |
Disulfide bonds
None
Other bonds (neither disulfide, covalent nor hydrogen bonds, e.g. Zinc–sulphur bond)
None
Non-standard residues
None
Sequence alignments
- Entity_assembly_ID (NMR): A vs Auth_asym_ID (model): A
--------10--------20--------30--------40
DAEFRHDSGYEVHHQKLVFFAEDVGSNKGAIIGLMVGGVV
||||||||||||||||||||||||||||||||||||||||
DAEFRHDSGYEVHHQKLVFFAEDVGSNKGAIIGLMVGGVV
Entity_assembly_ID (NMR): B vs Auth_asym_ID (model): B--------10--------20--------30--------40
DAEFRHDSGYEVHHQKLVFFAEDVGSNKGAIIGLMVGGVV
||||||||||||||||||||||||||||||||||||||||
DAEFRHDSGYEVHHQKLVFFAEDVGSNKGAIIGLMVGGVV
Entity_assembly_ID (NMR): C vs Auth_asym_ID (model): C--------10--------20--------30--------40
DAEFRHDSGYEVHHQKLVFFAEDVGSNKGAIIGLMVGGVV
||||||||||||||||||||||||||||||||||||||||
DAEFRHDSGYEVHHQKLVFFAEDVGSNKGAIIGLMVGGVV
Entity_assembly_ID (NMR): D vs Auth_asym_ID (model): D--------10--------20--------30--------40
DAEFRHDSGYEVHHQKLVFFAEDVGSNKGAIIGLMVGGVV
||||||||||||||||||||||||||||||||||||||||
DAEFRHDSGYEVHHQKLVFFAEDVGSNKGAIIGLMVGGVV
Entity_assembly_ID (NMR): E vs Auth_asym_ID (model): E--------10--------20--------30--------40
DAEFRHDSGYEVHHQKLVFFAEDVGSNKGAIIGLMVGGVV
||||||||||||||||||||||||||||||||||||||||
DAEFRHDSGYEVHHQKLVFFAEDVGSNKGAIIGLMVGGVV
Entity_assembly_ID (NMR): F vs Auth_asym_ID (model): F--------10--------20--------30--------40
DAEFRHDSGYEVHHQKLVFFAEDVGSNKGAIIGLMVGGVV
||||||||||||||||||||||||||||||||||||||||
DAEFRHDSGYEVHHQKLVFFAEDVGSNKGAIIGLMVGGVV
Entity_assembly_ID (NMR): G vs Auth_asym_ID (model): G--------10--------20--------30--------40
DAEFRHDSGYEVHHQKLVFFAEDVGSNKGAIIGLMVGGVV
||||||||||||||||||||||||||||||||||||||||
DAEFRHDSGYEVHHQKLVFFAEDVGSNKGAIIGLMVGGVV
Entity_assembly_ID (NMR): H vs Auth_asym_ID (model): H--------10--------20--------30--------40
DAEFRHDSGYEVHHQKLVFFAEDVGSNKGAIIGLMVGGVV
||||||||||||||||||||||||||||||||||||||||
DAEFRHDSGYEVHHQKLVFFAEDVGSNKGAIIGLMVGGVV
Entity_assembly_ID (NMR): I vs Auth_asym_ID (model): I--------10--------20--------30--------40
DAEFRHDSGYEVHHQKLVFFAEDVGSNKGAIIGLMVGGVV
||||||||||||||||||||||||||||||||||||||||
DAEFRHDSGYEVHHQKLVFFAEDVGSNKGAIIGLMVGGVV
Entity_assembly_ID (NMR): J vs Auth_asym_ID (model): J--------10--------20--------30--------40
DAEFRHDSGYEVHHQKLVFFAEDVGSNKGAIIGLMVGGVV
||||||||||||||||||||||||||||||||||||||||
DAEFRHDSGYEVHHQKLVFFAEDVGSNKGAIIGLMVGGVV
Entity_assembly_ID (NMR): K vs Auth_asym_ID (model): K--------10--------20--------30--------40
DAEFRHDSGYEVHHQKLVFFAEDVGSNKGAIIGLMVGGVV
||||||||||||||||||||||||||||||||||||||||
DAEFRHDSGYEVHHQKLVFFAEDVGSNKGAIIGLMVGGVV
Entity_assembly_ID (NMR): L vs Auth_asym_ID (model): L--------10--------20--------30--------40
DAEFRHDSGYEVHHQKLVFFAEDVGSNKGAIIGLMVGGVV
||||||||||||||||||||||||||||||||||||||||
DAEFRHDSGYEVHHQKLVFFAEDVGSNKGAIIGLMVGGVV
Chain assignments
| Entity_assembly_ID (NMR) | Auth_asym_ID (model) | Length | Unmapped | Conflict | Sequence coverage (%) |
|---|
| A | A | 40 | 0 | 0 | 100.0 |
| B | B | 40 | 0 | 0 | 100.0 |
| C | C | 40 | 0 | 0 | 100.0 |
| D | D | 40 | 0 | 0 | 100.0 |
| E | E | 40 | 0 | 0 | 100.0 |
| F | F | 40 | 0 | 0 | 100.0 |
| G | G | 40 | 0 | 0 | 100.0 |
| H | H | 40 | 0 | 0 | 100.0 |
| I | I | 40 | 0 | 0 | 100.0 |
| J | J | 40 | 0 | 0 | 100.0 |
| K | K | 40 | 0 | 0 | 100.0 |
| L | L | 40 | 0 | 0 | 100.0 |
Content subtype: combined_18127_2lmn.nef
| # | Content subtype | Saveframe | Status | # of rows (sets) | Experiment type | Sequence coverage (%) |
|---|
| 1 | Assigned chemical shifts | assigned_chem_shift_list_1 | OK | 110 | | 82.5 (chain: A, length: 40) |
| 1 | Distance restraints | CNS/XPLOR_distance_constraints_4 | Warning | 542 (1) | undefined | 55.0 (chain: A, length: 40), 65.0 (chain: B, length: 40), 65.0 (chain: C, length: 40), 57.5 (chain: D, length: 40), 62.5 (chain: E, length: 40), 65.0 (chain: F, length: 40), 65.0 (chain: G, length: 40), 65.0 (chain: H, length: 40), 57.5 (chain: I, length: 40), 55.0 (chain: J, length: 40), 62.5 (chain: K, length: 40), 65.0 (chain: L, length: 40) |
| 1 | Dihedral angle restraints | CNS/XPLOR_dihedral_3 | OK | 456 (456) | . | 65.0 (chain: A, length: 40), 65.0 (chain: B, length: 40), 65.0 (chain: C, length: 40), 65.0 (chain: D, length: 40), 65.0 (chain: E, length: 40), 65.0 (chain: F, length: 40), 65.0 (chain: G, length: 40), 65.0 (chain: H, length: 40), 65.0 (chain: I, length: 40), 65.0 (chain: J, length: 40), 65.0 (chain: K, length: 40), 65.0 (chain: L, length: 40) |
| 1 | RDC restraints | CNS/XPLOR_dipolar_coupling_2 | Warning | 184 (184) | . | 37.5 (chain: A, length: 40), 37.5 (chain: B, length: 40), 37.5 (chain: C, length: 40), 37.5 (chain: D, length: 40), 37.5 (chain: E, length: 40), 37.5 (chain: F, length: 40), 37.5 (chain: G, length: 40), 37.5 (chain: H, length: 40), 37.5 (chain: I, length: 40), 37.5 (chain: J, length: 40), 37.5 (chain: K, length: 40), 37.5 (chain: L, length: 40) |
Assigned chemical shifts
- Saveframe: assigned_chem_shift_list_1
- Total number of assignments
- 13C chemical shifts: 93
- 15N chemical shifts: 17
- Completeness of assignments
- Entity_assemble_ID: A
- All atoms
| Atom group | # of target shifts | # of assigned shifts | Completeness (%) |
|---|
| 13C chemical shifts | 177 | 93 | 52.5 |
| 15N chemical shifts | 43 | 17 | 39.5 |
- Backbone atoms
| Atom group | # of target shifts | # of assigned shifts | Completeness (%) |
|---|
| 13C chemical shifts | 80 | 66 | 82.5 |
| 15N chemical shifts | 40 | 17 | 42.5 |
- Side chain atoms
| Atom group | # of target shifts | # of assigned shifts | Completeness (%) |
|---|
| 13C chemical shifts | 97 | 27 | 27.8 |
| 15N chemical shifts | 3 | 0 | 0.0 |
- Methyl group atoms
| Atom group | # of target shifts | # of assigned shifts | Completeness (%) |
|---|
| 13C chemical shifts | 24 | 3 | 12.5 |
- Aromatic group atoms
| Atom group | # of target shifts | # of assigned shifts | Completeness (%) |
|---|
| 13C chemical shifts | 25 | 0 | 0.0 |
- Histogram of normalized assigned chemical shifts
- Random coil index and derived parameters (S2 and NMR RMSD)
Distance restraints
- Saveframe: CNS/XPLOR_distance_constraints_4
--------10--------20--------30--------40
DAEFRHDSGYEVHHQKLVFFAEDVGSNKGAIIGLMVGGVV
| ||||||||||| ||||||||||||||
...........V.HQKLVFFAEDV.SNKGAIIGLMVGGV
--------10--------20--------30---------
- Entity_assembly_ID: C, Chain length: 40, Sequence coverage: 65.0%
--------10--------20--------30--------40
DAEFRHDSGYEVHHQKLVFFAEDVGSNKGAIIGLMVGGVV
| ||||||||||| ||||||||||||||
...........V.HQKLVFFAEDV.SNKGAIIGLMVGGV
--------10--------20--------30---------
- Entity_assembly_ID: D, Chain length: 40, Sequence coverage: 57.5%
--------10--------20--------30--------40
DAEFRHDSGYEVHHQKLVFFAEDVGSNKGAIIGLMVGGVV
| ||| | | ||| ||||||||||||||
...........V.HQK.V.F.EDV.SNKGAIIGLMVGGV
--------10--------20--------30---------
- Entity_assembly_ID: E, Chain length: 40, Sequence coverage: 62.5%
--------10--------20--------30--------40
DAEFRHDSGYEVHHQKLVFFAEDVGSNKGAIIGLMVGGVV
| ||||||||||| || |||||||||||
...........V.HQKLVFFAEDV.SN.GAIIGLMVGGV
--------10--------20--------30---------
- Entity_assembly_ID: F, Chain length: 40, Sequence coverage: 65.0%
--------10--------20--------30--------40
DAEFRHDSGYEVHHQKLVFFAEDVGSNKGAIIGLMVGGVV
| ||||||||||| ||||||||||||||
...........V.HQKLVFFAEDV.SNKGAIIGLMVGGV
--------10--------20--------30---------
- Entity_assembly_ID: G, Chain length: 40, Sequence coverage: 65.0%
--------10--------20--------30--------40
DAEFRHDSGYEVHHQKLVFFAEDVGSNKGAIIGLMVGGVV
| ||||||||||| ||||||||||||||
...........V.HQKLVFFAEDV.SNKGAIIGLMVGGV
--------10--------20--------30---------
- Entity_assembly_ID: H, Chain length: 40, Sequence coverage: 65.0%
--------10--------20--------30--------40
DAEFRHDSGYEVHHQKLVFFAEDVGSNKGAIIGLMVGGVV
| ||||||||||| ||||||||||||||
...........V.HQKLVFFAEDV.SNKGAIIGLMVGGV
--------10--------20--------30---------
- Entity_assembly_ID: I, Chain length: 40, Sequence coverage: 57.5%
--------10--------20--------30--------40
DAEFRHDSGYEVHHQKLVFFAEDVGSNKGAIIGLMVGGVV
| ||| | | ||| ||||||||||||||
...........V.HQK.V.F.EDV.SNKGAIIGLMVGGV
--------10--------20--------30---------
- Entity_assembly_ID: J, Chain length: 40, Sequence coverage: 55.0%
--------10--------20--------30--------40
DAEFRHDSGYEVHHQKLVFFAEDVGSNKGAIIGLMVGGVV
| |||| | | || || |||||||||||
...........V.HQKL.F.A.DV.SN.GAIIGLMVGGV
--------10--------20--------30---------
- Entity_assembly_ID: K, Chain length: 40, Sequence coverage: 62.5%
--------10--------20--------30--------40
DAEFRHDSGYEVHHQKLVFFAEDVGSNKGAIIGLMVGGVV
| ||||||||||| || |||||||||||
...........V.HQKLVFFAEDV.SN.GAIIGLMVGGV
--------10--------20--------30---------
- Entity_assembly_ID: L, Chain length: 40, Sequence coverage: 65.0%
--------10--------20--------30--------40
DAEFRHDSGYEVHHQKLVFFAEDVGSNKGAIIGLMVGGVV
| ||||||||||| ||||||||||||||
...........V.HQKLVFFAEDV.SNKGAIIGLMVGGV
--------10--------20--------30---------
- Total number of restraints: 542
- Sequential restraints, | i - j | = 1 : 24
- Medium range restraints, 1 < | i - j | < 5 : 36
- Total hydrogen bond restraints: 204
- N...h_N (too far!): 36
- N...h_N: 48
- Symmetric restraints: 278
- Weight of restraints: 1.0 (542)
- Sequential restraints, | i - j | = 1 : 1.0 (24)
- Backbone-backbone: 1.0 (24)
- Medium range restraints, 1 < | i - j | < 5 : 1.0 (36)
- Backbone-side chain: 1.0 (36)
- Total hydrogen bond restraints: 1.0 (204)
- N...h_N (too far!): 1.0 (36)
- N...h_N: 1.0 (48)
- Symmetric restraints: 1.0 (278)
- Potential type of restraints: square-well-parabolic (542)
- Sequential restraints, | i - j | = 1 : square-well-parabolic (24)
- Backbone-backbone: square-well-parabolic (24)
- Medium range restraints, 1 < | i - j | < 5 : square-well-parabolic (36)
- Backbone-side chain: square-well-parabolic (36)
- Total hydrogen bond restraints: square-well-parabolic (204)
- N...h_N (too far!): square-well-parabolic (36)
- N...h_N: square-well-parabolic (48)
- Inter-chain: square-well-parabolic (120)
- O...H-x: square-well-parabolic (120)
- Symmetric restraints: square-well-parabolic (278)
- Range of distance target values: 1.8, 4.65 (Å)
- Histogram of distance target values
- Distance restraints per residue
- Chain_ID: A
- Chain_ID: B
- Chain_ID: C
- Chain_ID: D
- Chain_ID: E
- Chain_ID: F
- Chain_ID: G
- Chain_ID: H
- Chain_ID: I
- Chain_ID: J
- Chain_ID: K
- Chain_ID: L
- Distance restraints on contact map
- Chain_ID_1: A - Chain_ID_2: A
- Chain_ID_1: B - Chain_ID_2: B
- Chain_ID_1: C - Chain_ID_2: C
- Chain_ID_1: D - Chain_ID_2: D
- Chain_ID_1: E - Chain_ID_2: E
- Chain_ID_1: F - Chain_ID_2: F
- Chain_ID_1: G - Chain_ID_2: G
- Chain_ID_1: H - Chain_ID_2: H
- Chain_ID_1: I - Chain_ID_2: I
- Chain_ID_1: J - Chain_ID_2: J
- Chain_ID_1: K - Chain_ID_2: K
- Chain_ID_1: L - Chain_ID_2: L
- Chain_ID_1: A - Chain_ID_2: B
None
- Chain_ID_1: A - Chain_ID_2: C
None
- Chain_ID_1: A - Chain_ID_2: D
None
- Chain_ID_1: A - Chain_ID_2: E
- Chain_ID_1: A - Chain_ID_2: F
- Chain_ID_1: A - Chain_ID_2: G
None
- Chain_ID_1: A - Chain_ID_2: H
None
- Chain_ID_1: A - Chain_ID_2: I
None
- Chain_ID_1: A - Chain_ID_2: J
- Chain_ID_1: A - Chain_ID_2: K
None
- Chain_ID_1: A - Chain_ID_2: L
None
- Chain_ID_1: B - Chain_ID_2: C
- Chain_ID_1: B - Chain_ID_2: D
- Chain_ID_1: B - Chain_ID_2: E
None
- Chain_ID_1: B - Chain_ID_2: F
None
- Chain_ID_1: B - Chain_ID_2: G
- Chain_ID_1: B - Chain_ID_2: H
None
- Chain_ID_1: B - Chain_ID_2: I
None
- Chain_ID_1: B - Chain_ID_2: J
None
- Chain_ID_1: B - Chain_ID_2: K
- Chain_ID_1: B - Chain_ID_2: L
- Chain_ID_1: C - Chain_ID_2: D
- Chain_ID_1: C - Chain_ID_2: E
None
- Chain_ID_1: C - Chain_ID_2: F
None
- Chain_ID_1: C - Chain_ID_2: G
None
- Chain_ID_1: C - Chain_ID_2: H
- Chain_ID_1: C - Chain_ID_2: I
None
- Chain_ID_1: C - Chain_ID_2: J
None
- Chain_ID_1: C - Chain_ID_2: K
None
- Chain_ID_1: C - Chain_ID_2: L
- Chain_ID_1: D - Chain_ID_2: E
None
- Chain_ID_1: D - Chain_ID_2: F
None
- Chain_ID_1: D - Chain_ID_2: G
None
- Chain_ID_1: D - Chain_ID_2: H
None
- Chain_ID_1: D - Chain_ID_2: I
- Chain_ID_1: D - Chain_ID_2: J
None
- Chain_ID_1: D - Chain_ID_2: K
None
- Chain_ID_1: D - Chain_ID_2: L
None
- Chain_ID_1: E - Chain_ID_2: F
- Chain_ID_1: E - Chain_ID_2: G
- Chain_ID_1: E - Chain_ID_2: H
None
- Chain_ID_1: E - Chain_ID_2: I
None
- Chain_ID_1: E - Chain_ID_2: J
None
- Chain_ID_1: E - Chain_ID_2: K
- Chain_ID_1: E - Chain_ID_2: L
None
- Chain_ID_1: F - Chain_ID_2: G
- Chain_ID_1: F - Chain_ID_2: H
- Chain_ID_1: F - Chain_ID_2: I
None
- Chain_ID_1: F - Chain_ID_2: J
None
- Chain_ID_1: F - Chain_ID_2: K
None
- Chain_ID_1: F - Chain_ID_2: L
- Chain_ID_1: G - Chain_ID_2: H
- Chain_ID_1: G - Chain_ID_2: I
- Chain_ID_1: G - Chain_ID_2: J
None
- Chain_ID_1: G - Chain_ID_2: K
None
- Chain_ID_1: G - Chain_ID_2: L
None
- Chain_ID_1: H - Chain_ID_2: I
- Chain_ID_1: H - Chain_ID_2: J
None
- Chain_ID_1: H - Chain_ID_2: K
None
- Chain_ID_1: H - Chain_ID_2: L
None
- Chain_ID_1: I - Chain_ID_2: J
None
- Chain_ID_1: I - Chain_ID_2: K
None
- Chain_ID_1: I - Chain_ID_2: L
None
- Chain_ID_1: J - Chain_ID_2: K
- Chain_ID_1: J - Chain_ID_2: L
- Chain_ID_1: K - Chain_ID_2: L
Dihedral angle restraints
- Saveframe: CNS/XPLOR_dihedral_3
--------10--------20--------30--------40
DAEFRHDSGYEVHHQKLVFFAEDVGSNKGAIIGLMVGGVV
||||||||||||||| |||||||||||
........GYEVHHQKLVFFAED......AIIGLMVGGVV
- Entity_assembly_ID: C, Chain length: 40, Sequence coverage: 65.0%
--------10--------20--------30--------40
DAEFRHDSGYEVHHQKLVFFAEDVGSNKGAIIGLMVGGVV
||||||||||||||| |||||||||||
........GYEVHHQKLVFFAED......AIIGLMVGGVV
- Entity_assembly_ID: D, Chain length: 40, Sequence coverage: 65.0%
--------10--------20--------30--------40
DAEFRHDSGYEVHHQKLVFFAEDVGSNKGAIIGLMVGGVV
||||||||||||||| |||||||||||
........GYEVHHQKLVFFAED......AIIGLMVGGVV
- Entity_assembly_ID: E, Chain length: 40, Sequence coverage: 65.0%
--------10--------20--------30--------40
DAEFRHDSGYEVHHQKLVFFAEDVGSNKGAIIGLMVGGVV
||||||||||||||| |||||||||||
........GYEVHHQKLVFFAED......AIIGLMVGGVV
- Entity_assembly_ID: F, Chain length: 40, Sequence coverage: 65.0%
--------10--------20--------30--------40
DAEFRHDSGYEVHHQKLVFFAEDVGSNKGAIIGLMVGGVV
||||||||||||||| |||||||||||
........GYEVHHQKLVFFAED......AIIGLMVGGVV
- Entity_assembly_ID: G, Chain length: 40, Sequence coverage: 65.0%
--------10--------20--------30--------40
DAEFRHDSGYEVHHQKLVFFAEDVGSNKGAIIGLMVGGVV
||||||||||||||| |||||||||||
........GYEVHHQKLVFFAED......AIIGLMVGGVV
- Entity_assembly_ID: H, Chain length: 40, Sequence coverage: 65.0%
--------10--------20--------30--------40
DAEFRHDSGYEVHHQKLVFFAEDVGSNKGAIIGLMVGGVV
||||||||||||||| |||||||||||
........GYEVHHQKLVFFAED......AIIGLMVGGVV
- Entity_assembly_ID: I, Chain length: 40, Sequence coverage: 65.0%
--------10--------20--------30--------40
DAEFRHDSGYEVHHQKLVFFAEDVGSNKGAIIGLMVGGVV
||||||||||||||| |||||||||||
........GYEVHHQKLVFFAED......AIIGLMVGGVV
- Entity_assembly_ID: J, Chain length: 40, Sequence coverage: 65.0%
--------10--------20--------30--------40
DAEFRHDSGYEVHHQKLVFFAEDVGSNKGAIIGLMVGGVV
||||||||||||||| |||||||||||
........GYEVHHQKLVFFAED......AIIGLMVGGVV
- Entity_assembly_ID: K, Chain length: 40, Sequence coverage: 65.0%
--------10--------20--------30--------40
DAEFRHDSGYEVHHQKLVFFAEDVGSNKGAIIGLMVGGVV
||||||||||||||| |||||||||||
........GYEVHHQKLVFFAED......AIIGLMVGGVV
- Entity_assembly_ID: L, Chain length: 40, Sequence coverage: 65.0%
--------10--------20--------30--------40
DAEFRHDSGYEVHHQKLVFFAEDVGSNKGAIIGLMVGGVV
||||||||||||||| |||||||||||
........GYEVHHQKLVFFAED......AIIGLMVGGVV
- Total number of restraints: 456
- Phi angle constraints: 228
- Psi angle constraints: 228
- Total number of restraints per polymer type: 456
- Weight of restraints: 1.0 (456)
- Phi angle constraints: 1.0 (228)
- Psi angle constraints: 1.0 (228)
- Potential type of restraints: square-well-parabolic (456)
- Phi angle constraints: square-well-parabolic (228)
- Psi angle constraints: square-well-parabolic (228)
- Plot of Phi-Psi angle restraints
- Dihedral angle restraints per residue
- Chain_ID: A
- Chain_ID: B
- Chain_ID: C
- Chain_ID: D
- Chain_ID: E
- Chain_ID: F
- Chain_ID: G
- Chain_ID: H
- Chain_ID: I
- Chain_ID: J
- Chain_ID: K
- Chain_ID: L
RDC restraints
- Saveframe: CNS/XPLOR_dipolar_coupling_2
- Status: Warning
- Experiment type: .
- Sequence coverage of experimental data
- Entity_assembly_ID: A, Chain length: 40, Sequence coverage: 37.5%
- Entity_assembly_ID: B, Chain length: 40, Sequence coverage: 37.5%
- Entity_assembly_ID: C, Chain length: 40, Sequence coverage: 37.5%
- Entity_assembly_ID: D, Chain length: 40, Sequence coverage: 37.5%
- Entity_assembly_ID: E, Chain length: 40, Sequence coverage: 37.5%
- Entity_assembly_ID: F, Chain length: 40, Sequence coverage: 37.5%
- Entity_assembly_ID: G, Chain length: 40, Sequence coverage: 37.5%
- Entity_assembly_ID: H, Chain length: 40, Sequence coverage: 37.5%
- Entity_assembly_ID: I, Chain length: 40, Sequence coverage: 37.5%
- Entity_assembly_ID: J, Chain length: 40, Sequence coverage: 37.5%
- Entity_assembly_ID: K, Chain length: 40, Sequence coverage: 37.5%
- Entity_assembly_ID: L, Chain length: 40, Sequence coverage: 37.5%
- Total number of restraints: 184
- CE-CE bond vectors: 10
- C-C bond vectors: 30
- H-N bond vectors: 144
- Potential type of restraints: undefined (184)
- CE-CE bond vectors: undefined (10)
- C-C bond vectors: undefined (30)
- H-N bond vectors: undefined (144)
- Range of observed RDC values: 100.0, -100.0 (Hz)
- RDC restraints per residue